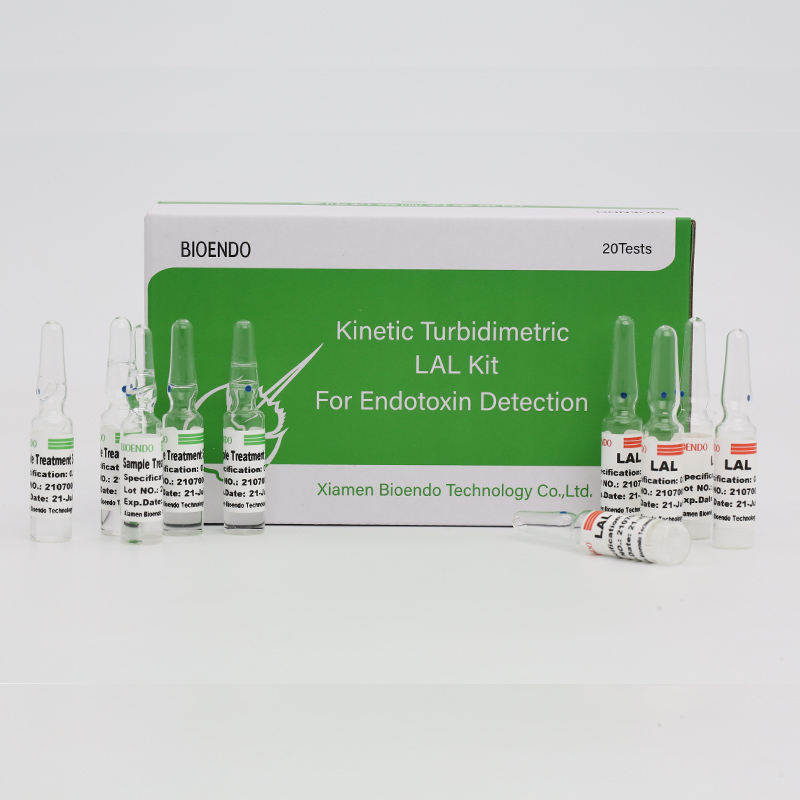
Endotoxin Asssay Kit pro Plasma Humanum
Endotoxin Asssay Kitad Humanum Plasma
1. Product Information
CFDA purgatumOrci diagnostica Endotoxin primordium ornamentuminhumanus plasma quantificat endotoxin level.Endotoxin maior pars est muri cellae bacteria Negativae et est potissima mediatrix sepsis microbialis.Gradus endotoxini elevati saepe febrem inducere possunt, mutationes in albo sanguine cellulis comitibus, in quibusdam casibus, concussio cardiovascularis.Fundatur e factor Cpathway in limul Polyphemi (cancri equina) test.In motu microplate lectoris et programmatis endotoxini primordium, Endotoxin primordium ornamentum endotoxinum in plano plasmatis hominis minus quam una hora detegit.Ornamentum venit cum plasma prae-tractationis gerentis, quod factores inhibitionis in plasma per endotoxin primordium eliminat.
2. Product Parameter
Temptare range: 0.01-10 EU/ml
3. Product Feature ac Application
Venit cum solutionibus plasmatis praetractationis, factores inhibitionis in plasmate humano eliminat.
Nota:
Lyophilised Amebocyte Lysate (LAL) gerens a Bioendo factorum factum est ex amebocyte lysate derivato sanguine cancri equini.
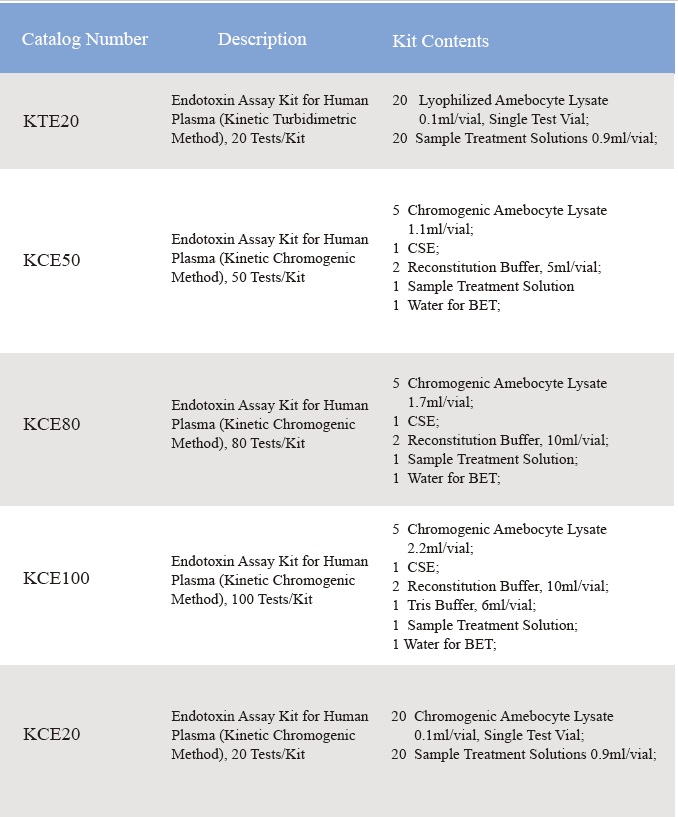
Sensus Lyophilised Amebocyte Lysate et potentia Imperii Latin Endotoxin tentantur contra USP Reference Standard Endotoxin.Lyophilised Amebocyte Lysate iodi rhoncus cum productum instructione veniunt, Testimonia Analysis, MSDS.