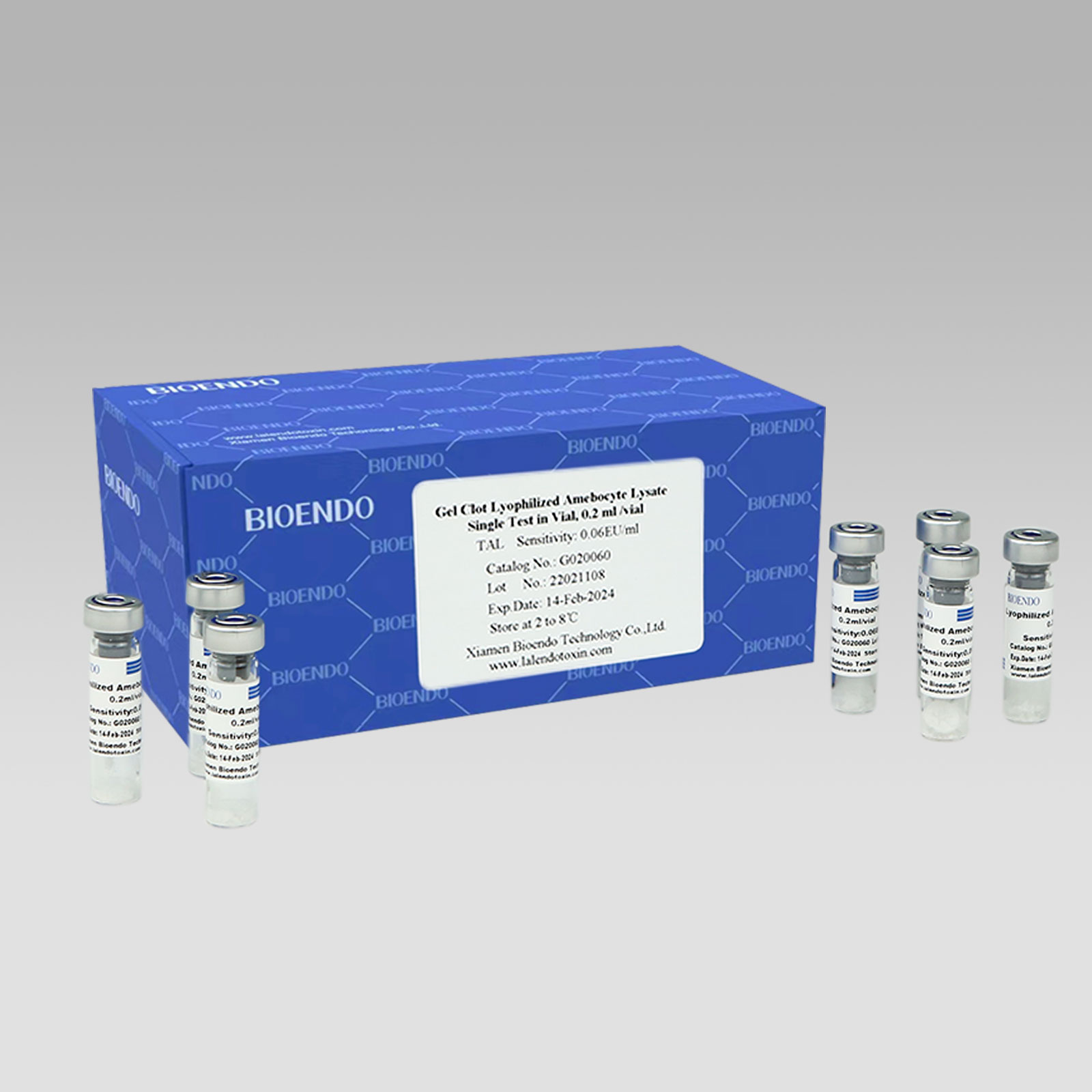
gel Clot Lyophilized Amebocyte Lysate Singulus Test in Vial
Gel Clot LyophilizedAmebocyte LysateUnius Test in Vial
1. Product Information
Gel Clot LyophilizedAmebocyte LysateUnius Testin Vial Continete endotoxin-specialis Amebocyte Lysate quae includit inhibitorem beta-glucan in formula et non agere cum beta-glucan.Pro Singulo Testi nostro in Vial, specimen phialae directe addere potes.Hoc modo non debes Amebocyte Lysate primo renovare, et quot phialas utantur singulis diebus ad evitandas solitudines iudicabis.Endotoxinum liberum fistulam ad dilutionem CSE necessariam est.Operatioendotoxin deprehensioper electionem Bioendo Gel Clot Lyophilised Amebocyte Lysate Single Test in Vial conforme ad USP, EP.
2. Product Parameter
Gel concretum primordium unum test phialam vitream
Sensus: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml
3.Product Feature et Application
unus gradusendotoxin deprehensio,
sine sumptuosusendotoxin primordiuminstrumenta;
Apta forfinis-productumendotoxin temptationisante productum dimisit;
Productum sensitivum normatum secundum pharmacopoeam US criterium et criterium pharmacopoeiae Sinarum.
Nota:
Lyophilised Amebocyte Lysate (LAL) gerens a Bioendo fabricatum factum est ex lysate amebocytarum (cellulae albae sanguinis) ex cancri solea.
| Catalogus numerus | Sensus (EU/ml) (IU/ml) | Probat/Vial | fialas/Pack |
| G002015 | 0.014 | 1 | 50 |
| G02030 | 0.03 | 1 | 50 |
| G020060 | 0.06 | 1 | 50 |
| G020125 | 0.125 | 1 | 50 |
| G020250 | 0.25 | 1 | 50 |
| G020500 | 0.5 | 1 | 50 |
Product Condition
Sensus Lyophilised Amebocyte Lysate et potentia Imperii Latin Endotoxin tentantur contra USP Reference Standard Endotoxin.Lyophilised Amebocyte Lysate iodi rhoncus cum productum instructione veniunt, certificatorium Analyseos.
Quare potissimum gel concretum primordium G02 ornamentum excerpsit;
1. Frugi maxime ac communiter probandi iodi deprehendendi endotoxin.
2. Unius experimentum in phiala unius gradus contaminationis periculum minuunt.
3. Gel concretum tentamen unius phialae vitreae test non eget lectori microplate urbano.
4. Servans endotoxin liberum fistulam cum G02 seriem adhibens ad endotoxin probandi in processu operationis endotoxini testi tentandi.